Scientists
raise possibility of vaccine for Alzheimer's disease
Deborah Josefson
, San Francisco
An
experimental vaccine directed against amyloid, a protein implicated in
Alzheimer's disease, has shown promise in animal trials (Nature 1999;400:173-7).
The vaccine may lead to an effective treatment.
Scientists
at Elan Pharmaceuticals developed a transgenic mouse model of Alzheimer's
disease by injecting the rodent with a mutant form of the human amyloid
precursor protein. The mutant amyloid precursor protein gene, which occurs in a
number of familial forms of Alzheimer's disease, leads to an overexpression of
the amyloid <Immagine: beta > peptide, the principal constituent of
amyloid plaque in the disease.
Amyloid
plaques consist of insoluble aggregates of amyloid protein and are thought to be
involved in neuronal cell death.
The
transgenic mice developed amyloid plaques in their brains in a manner specific
to age and brain region, mimicking the changes seen in human forms of
Alzheimer's disease.
The
researchers then sought to see if immunisation with a fragment of amyloid
protein would modify the disease in their affected mice. Accordingly, mice were
immunised with a 42 amino acid segment of the amyloid B protein.
One
group of mice was immunised at 6 weeks of age, when the neuropathological
hallmarks of Alzheimer's disease are not yet present, and a second group at 11 months
of age, when amyloid deposition is already prominent. Two main experiments were
then conducted. In the first experiment (with the mice immunised at 6 weeks
old), three control groups were used for comparison: one group received saline
vaccinations, a second was left untreated, and the third was immunised with
another plaque associated protein (serum amyloid protein).
At
the age of 13 weeks, the groups vaccinated at 6 weeks were killed and
their brains examined. Seven out of the nine mice treated with the b amyloid
protein had no detectable plaques, whereas the other groups showed age related
plaque accumulation.
In
the second experiment (with the mice immunised at 11 months old) two
control groups from the same litter were left untreated. At 18 months the
vaccinated mice showed significantly less plaque formation than their 18 month
old controls. They also had less gliosis and neuritic dystrophy. Even more
striking, however, was the finding that these mice also had less plaque
formation than younger untreated control mice, those at 12 months old.
This
suggests that immunisation with the amyloid b protein facilitated the removal of
amyloid plaque, probably by an antibody mediated immune attack.
Commenting
on the study, Dale Schenk, the chief investigator on the project, said:
"When we examined that group at 18 months ... we expected to see
widespread brain pathology [but] it had been halted in its tracks.
The
brain tissue looked essentially like [that of] the original 11 month old
animal and in fact looked somewhat better. This suggested to us that the vaccine
had potential for treatment."
The
researchers said that the vaccine did not have any detectable side effects in
mice.
Although
the results are promising, it is not known if the findings are applicable to
humans. Whether amyloid deposition is the cause or the effect of Alzheimer's
disease is still widely debated.
Furthermore,
although the mice developed plaques, they lacked other features of the disease,
such as neurofibrillary
tangles,
and cognitive decline.
A
spokesman for Elan Pharmaceuticals said that the company plans to submit an
application to the US Food and Drug Administration and hopes to begin human
clinical trials by the end of next year.
Science,
medicine, and the future: Alzheimer's disease Colin L Masters, head,a Konrad
Beyreuther, director
There
is a noticeable air of optimism in the research community studying Alzheimer's
disease. This because the molecular basis of Alzheimer's disease and other
neurodegenerative conditions, such as Parkinson's and Huntington's diseases, is
rapidly being elucidated. From these molecular insights, it is likely that
effective therapeutic strategies will be developed within the next 10 years.
Future treatment will probably be based on combination therapies—such as
neurotransmitter replacement combined with a drug to protect against the toxic
effect of Aß amyloid—tailored to the genetic profile of an individual.
Assuming proved efficacy and safety, these forms of treatment are likely to be
as widespread and acceptable as cholesterol lowering treatment is today.
In
the past few years there has been an avalanche of knowledge surrounding the
genesis of Aß amyloid plaques, one of the principal pathological hallmarks of
this disease (see fig 1). In the absence of a serious candidate for the pathway
leading to the neurofibrillary tangle, the other major pathological lesion in
Alzheimer's disease (fig 1), the focus will probably remain on amyloid plaques.
Strong genetic risk factors have been identified for Alzheimer's disease, all of
which interact directly or indirectly with the Aß amyloid pathway. Undoubtedly,
other genetic factors remain to be discovered, some of which might open the door
to the neurofibrillary tangle. More importantly, the major environmental risk
factors for Alzheimer's disease remain elusive. This is not surprising, given
the relatively few analytical epidemiological studies that have been conducted
and the difficulty of case ascertainment, particularly in the early stages of
the disease.
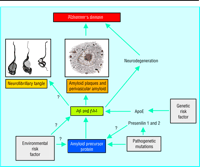
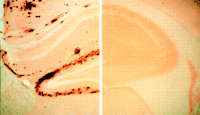
Fig 1 The central pathway leading to Alzheimer's disease involves the
processing of amyloid precursor protein into Aß amyloid, which accumulates as
amyloid plaques or perivascular amyloid. Concomitantly, degeneration occurs in
neurons and their processes, leading to neurofibrillary tangles. (Images are
from Spielmeyer's Histopathology of the Nervous System, 1922.) Mutations in the
gene encoding amyloid precursor protein can cause Alzheimer's disease, as do
mutations in the presenilin genes. Inheritance of particular polymorphisms in
genes such as ApoE also can increase susceptibility for Alzheimer's disease.
Major environmental risk factors for Alzheimer's disease remain to be determined
We
review briefly where the research is heading and give some predictions on where
our concepts might lie a decade from now (for more details see recent reviews1 2
3).
Many
lines of evidence confirm that the generation of Aß amyloid from the amyloid
precursor protein is the central pathway in Alzheimer's disease (see fig 2). The
clinching evidence has come from the recognition that rare genetic mutations in
the gene encoding amyloid precursor protein actually cause Alzheimer's disease
at an early age (onset before 65 years).

Fig 2 (a) Cleavage of amyloid precursor protein (APP) by enzymes (secretases)
release the Aß amyloidogenic fragment. (b) The critical region of amyloid
precursor protein shown schematically in the one letter amino acid code. The
secretases act at three principal sites (<Immagine: {alpha}>, ß, and <Immagine:
{gamma}>). Mutations in the gene for amyloid precursor protein at these sites
can adversely affect the action of secretases: mutations towards the NH2
terminus increase the absolute rate of ß-secretion, while mutations near the
COOH terminus affect the ratio of Aß42 to Aß40. The Aß42 forms are more
damaging for nerve cells
Possible
futures
Genotype
screening, analysis, and counselling
Presymptomatic
diagnosis
Rational
preventive treatment—drug based or gene based
Advice
on preventive measures—changes to lifestyle to avoid known environmental risk
factor(s)
Drugs
targeting the amyloidogenic pathway to modify the course of the disease
Amyloid
precursor protein is a normal transmembrane glycoprotein that is widely
expressed in the body, but particularly in brain and platelets. Its function
remains uncertain: mice lacking the protein seem largely normal but have subtle
defects in synaptic function. Expression of the gene for the protein is closely
regulated and responds quickly to a wide variety of cellular stresses and
exogenous factors (including trauma, oestrogens, and certain metal ions).
Generated in the endoplasmic reticulum, amyloid precursor protein is sent to the
Golgi apparatus for glycation before export to the cell surface.
At
critical points of its biogenesis, amyloid precursor protein is subjected to
enzymatic proteolytic cleavages, which in concert generate the Aß peptides (fig
2). These enzymes, termed secretases, release the amyloid precursor protein from
the cell membrane and thereby affect the proportion of the protein that remains
on the cell surface or is released into the extracellular milieu. The Aß
peptides encompass part of the hydrophobic transmembrane domain. The exact
cleavage sites of the <Immagine: {gamma}>-secretases are important, since
the length of the hydrophobic tail of the Aß peptide may be a crucial factor
determining its aggregation and toxicity. Thus, the shorter Aß40 is the species
most often identified in non-neuronal cells and has less tendency to aggregate
than the longer Aß42: it is this longer Aß42 that is found at the centre of
amyloid plaques. Neuronal cells have a propensity to make the longer forms,
probably in a different cellular compartment (the endoplasmic reticulum). How
either form is released from the cell remains uncertain.
Once
released from the cell, Aß peptides aggregate into amyloid fibrils. The rates
of deposition and clearance of Aß from the brain may be critical determinants
in establishing disease. The exact mechanisms by which Aß exerts its toxicity
or adverse "gain of function" is under intense scrutiny.
Other
chronic degenerative diseases of aging nervous system
Recent
elucidation of a variety of gene mutations causing diverse chronic
neurodegenerative diseases point to a common mechanism—the toxic gain of
function of small, relatively insoluble, protein polymers (see box). If further
research confirms and extends this line of reasoning, Alzheimer's disease may
eventually be seen as only one example of a process in which an abnormally
shaped molecule accumulates in the brain and causes neuronal damage. In that
case, a treatment developed for the toxic effect of the polyglutamine expression
of the abnormal gene in Huntington's disease might be relevant for one or all of
the other neurodegenerative diseases.
Neurodegenerative
diseases associated with abnormal protein conformations (toxic gain of function)
Disease
•Alzheimer's
disease
•Creutzfeldt-Jakob
disease
•Amyotrophic
lateral sclerosis
•Parkinson's
disease
•Huntington's
disease
•Machado-Joseph
disease
Gene
product
Amyloid
precursor protein and Aß amyloid
Prion
protein
Superoxide
dismutase
{alpha}>-synuclein
Huntingtin
Ataxin-3
The
discovery of the presenilin family of genes has been a major breakthrough for
research.4 Together with mutations in amyloid precursor protein, mutations in
these presenilin genes also cause early onset of Alzheimer's disease and
probably act directly through the amyloidogenic pathway. We have now identified
about half of all the causative genes (responsible for possibly 10-20% of all
cases of Alzheimer's disease). Over the next decade, it is highly likely that
the remaining genes will be discovered, particularly in view of the rapid
progress in mapping and sequencing the human genome.
In
contrast with the causative genetic mutations, genetic risk factors are emerging
as important contributors to the occurrence of sporadic Alzheimer's disease
(responsible for 80-90% of all cases). The first to be identified, the ApoE gene
on chromosome 19, has provided clues to the likely size of effect of these
"public" genetic polymorphisms in a complex disease. Thus, inheritance
of the ApoE-<Immagine: {epsilon}>4 allele may increase the risk for
Alzheimer's disease by up to eightfold. In the near future other genetic loci
that act as susceptibility factors for Alzheimer's disease will undoubtedly be
discovered. For example, there is much current interest in loci on chromosome
12. These discoveries will bring forward the emerging field of pharmacogenetics,
in which treatments and preventive strategies will be tailored to an
individual's genetic profile.
Environmental
factors might be expected to have a role in causing Alzheimer's disease, in
common with all multifactorial complex diseases, but, surprisingly, none has yet
been convincingly identified. Estimates of relative risk indicate that factors
such as low education, head trauma, smoking, concomitant vascular disease,
diabetes, and the menopause have modest or inconsequential effects. Is there a
major environmental risk factor still waiting to be discovered by some
enterprising epidemiologist? Could some subtle factor in the Western diet or
lifestyle be uncovered through a more thorough understanding of the
amyloidogenic pathway? For example, we know that metal ions (such as copper and
zinc) interact adversely with amyloid precursor protein and Aß, and evidence is
emerging that oxidative stress mediated by hydroxyl radicals could underlie the
basis of Aß toxicity. These clues may provide the impetus for future
epidemiological studies.
A
major impediment to the development of rational treatments has been the lack of
an authentic and practical small animal model of Alzheimer's disease.
Fortunately, this seems to have been solved by the development of various
transgenic mouse models, which are progressively looking more like the human
disease. The latest are based on the overexpression of amyloid precursor protein
combined with the effects of the causative human mutations.5 The next step may
be to modulate the strain background of the mice or to introduce another
transgene to replicate the full human phenotype. Progress in this area has been
so rapid that there is every reason to believe that an effective mouse model
will soon be available.
In
the past decade, much has been learned about the conduct of clinical trials by
which the efficacy of any proposed treatment for Alzheimer's disease can be
assessed. The licensing of compounds such as tacrine, donepezil, and rivastigmin
have set standards by which all future drugs will be judged. There are currently
four drugs awaiting approval and more than 16 drugs undergoing phase III
clinical evaluation. Most are directed at the cholinergic system. Drugs
specifically targeting the amyloidogenic pathway (see box) are only now
beginning to emerge in a preclinical setting.
Therapeutic
targets in the amyloidogenic pathway
•Inhibit
Aß forming enzymes
•Redirect
processing of amyloid precursor protein away from Aß42
•Inhibit
aggregation or promote dissolution of Aß
•Ameliorate
toxicity of Aß
•Suppress
reactive responses to Aß toxicity
Looking
ahead to the next decade, it is likely that a comprehensive package of genotypic
analysis, presymptomatic diagnosis, and advice on preventive measures will be
advocated (see box), with the use of a combination of drugs that effectively
modify the course of the disease. Perhaps it is too much to expect any form of
curative treatment by the year 2008, but the underlying concepts and principles
for preventing the amyloidogenic processes from damaging neurons is
straightforward and eminently amenable to intervention. Current estimates of the
economic and social costs of Alzheimer's disease vary widely within and between
countries, but all agree on the immense size of the problem and that it will
increase dramatically over the next decade. The cost effectiveness of any
preventive treatment is potentially enormous. In contrast with the small effect
of today's symptomatic treatments,6 future strategies may alleviate a burden
that threatens most families; up to 25% of a family's annual income is required
to care for a member with Alzheimer's disease.7 As the average duration of the
illness is 10 years, it is relatively easy to derive a rough estimate of the
economic impact of the disease. Since a large proportion of the population is at
risk of developing Alzheimer's disease (possibly over half), an effective drug
based preventive treatment would justify universal screening (probably beginning
at ages 40-50 years, when amyloid plaques are starting to appear in the temporal
cortex).
Managing
Alzheimer's disease in the year 2008
•Genotype
screening, analysis, and counselling
•Presymptomatic
diagnosis
•Rational
preventive treatment—drug based or gene based
•Changes
to lifestyle—avoiding the environmental risk factor(s)
References
1.Hardy
J. Amyloid, the presenilins and Alzheimer's disease. Trends Neurosci
1997;20:154-9. [Medline] 2.Selkoe DJ. Amyloid ß-protein and the genetics of
Alzheimer's disease. J Biol Chem 1996;271:18295-8. [Full Text] 3.Yankner BA.
Mechanisms of neuronal degeneration in Alzheimer's disease. Neuron
1996;16:921-32. [Medline] 4.Kim T-W, Tanzi RE. Presenilins and Alzheimer's
disease. Curr Opin Neurobiol 1997;7:683-8. [Medline] 5.Struchler-Pierrat C,
Abramowski D, Duke M, Wiederhold K-H, Mistl C, Rothacher S, et al. Two amyloid
precursor protein transgenic mouse models with Alzheimer disease-like pathology.
Proc Natl Acad Sci USA 1997;94:13287-92. [Full Text] 6.Wimo A, Karlsson G,
Nordberg A, Winblad B. Treatment of Alzheimer disease with tacrine: a
cost-analysis model. Alzheimer Dis Assoc Disord 1997;11:191-200. [Medline]
7.Cavallo MC, Fattore G. The economic burden of Alzheimer disease on families in
the Lombardy region of Italy. Alzheimer Dis Assoc Disord 1997;11:184-90
Please follow the links or contact us for further information.
|